An Australasian Bone Marrow Transplant Registry (ABMTR) Study of the Trends and Outcomes of Allogeneic Haematopoietic Stem Cell Transplantation (HSCT) in Hodgkin Lymphoma between 2009-2019: Relapse remains the most common cause of death post transplantation
Introduction:
Hodgkin Lymphoma (HL) is an eminently curable disease, with 80% of cases achieving cure with first line therapies. There are a subset of patients who relapse and require salvage therapy including autologous stem cell transplant and more recently novel agents such as brentuximab vedotin (BV) and the PD-1 inhibitors. The latter are less toxic and achieve durable responses but are not considered curative for most (LaCasce et al., 2019). In Australia BV and PD-1 inhibitors were approved in December 2013 and September 2017 respectively. Allogeneic HSCT offers a graft vs lymphoma (GVL) effect that may contribute to long term survival in some patients (Peggs et al., 2005). The introduction of reduced intensity conditioning (RIC) has seen improved outcomes with an OS of 67% (59-74%) and Progression Free Survival (PFS)of 45% (35-56%) (Rashidi et al., 2016)
Patients and methods:
Data was collected through the Australasian Bone Marrow Transplant Recipient Registry (ABMTRR) for patients receiving a first allogeneic transplant for HL between 2009 and 2019. The Kaplan Meier method was used to calculate OS and PFS with log rank test for comparison. Multivariable Cox proportional hazards models were used to identify associations with OS. We divided the patients into 5 year cohorts to compare transplant outcomes over time.
Results:
A total of 149 patients from 16 sites in Australia and New Zealand were included. The median age at time of transplant was 31 years (range 19-61) and the majority were male (60%). Seventy-five percent of patients had undergone previous autologous HSCT with data missing for 22%. Median follow up was 75 months (range 4.7-137.1). Forty five percent of patients were in complete remission (CR), 34% in partial remission and 15% relapsed/primary refractory (RR) at the time of HSCT with information missing in 4%. The majority of donors were matched unrelated donors (47%) and sibling donors were used for 37% of patients, haploidentical in 11% and umbilical cord blood in 5%. Reduced intensity conditioning was used in 86% of patients and in vivo T cell depletion with ATG or alemtuzumab was used in 27%.
Acute GVHD occurred in 53/149 (30%) of which 31% was grade III-IV. In patients who survived beyond 100 days, the incidence of chronic GVHD was 38%, of which 53% was preceded by some form of aGVHD. Non-relapse mortality (NRM) at 100 days was 8% with 5/12 of these patients dying from aGVHD.
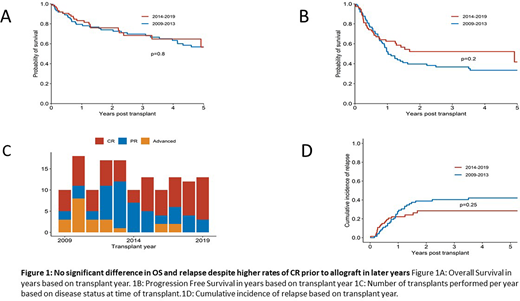
Two-year OS and PFS were 75% and 49% respectively. A period effect was not detected with no significant difference in OS (p=0.8) nor PFS (0.2) based on transplant year (figure 1a & 1b). Multivariate analysis of factors associated with OS identified age at transplant of >40 (HR 3.24, 95% CI 1.71-6.15, p<0.001) and RR disease at time of transplant (HR 3.07, 95% CI 1.44-6.54, p=0.004) with a higher risk of death.
The numbers of HSCT performed each year are illustrated in figure 1c, with a larger proportion of patients in CR from 2014 onward. Post-transplant relapse occurred in 38% of patients (figure 1d)with a median time to relapse of 8.5 months (range 0.2 -42). Forty-eight percent (27/56) of patients who relapsed post HSCT were in CR at the time of HSCT. Of those who relapsed, 37% died due to progressive disease with no evidence of chronic GVHD. Relapse was the most common cause of death (37%)
Conclusion:
Although the rates of HSCT for HL in Australia and New Zealand have not varied over the past decade despite the availability of novel agents, there is a larger proportion of patients in CR prior to transplant. Survival outcomes for HL post HSCT are comparable to those reported internationally. Despite a higher percentage of patients transplanted in CR in later years, relapse post HSCT remains the major cause of death. Further studies to examine strategies to prevent or treat relapse of HL post-allograft are needed.
Sharplin:Novartis: Other: FUnded to attend Australian Haematology Conference . Di Ciaccio:Jansen: Honoraria, Other: travel and accomodation grant. Spencer:AbbVie, Celgene, Haemalogix, Janssen, Sanofi, SecuraBio, Specialised Therapeutics Australia, Servier and Takeda: Consultancy; Amgen, Celgene, Haemalogix, Janssen, Servier and Takeda: Research Funding; AbbVie, Amgen, Celgene, Haemalogix, Janssen, Sanofi, SecuraBio, Specialised Therapeutics Australia, Servier and Takeda: Honoraria; Celgene, Janssen and Takeda: Speakers Bureau. Greenwood:Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Hamad:Abbvie: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal